Migraine curtails everyday life for unreasonably many people
Migraine constitutes a significant burden, not only to the individual suffering from this disabling disease but also to the society via sick leaves and use of health care resources. Two recent registry studies outline the burden of migraine in a Nordic setting and highlight the potential of erenumab, the first monoclonal antibody1 developed for prevention of migraine, to lower the burden of headache-related societal costs.
Since 1990, migraine has been one of the top five leading causes for disability worldwide, and in 2016 it was ranked the number one cause in people below 502. An extensive Finnish study1 recently put these global numbers into a Nordic perspective. Here, data from 17,623 individuals with migraine from a registry of occupational health care users* not only confirmed that migraine limits the lives of people living with the disease but also demonstrated its broader impact at a societal level3.
The highest proportion of people living with migraine were found among women of prime working age. In line with the disabling nature of the disease, people with migraine had almost twice as many sick days (17.6 per year) as compared to matching controls without migraine (9.7 per year). People living with migraine use of healthcare resources was also markedly higher (14.4 visits per year) as compared to that of people without migraine (8.5 visits per year)4.

A closer look at the data reveals some discrepancies between the individual subgroups of migraine patients. Whereas most of the migraine patients who were not on medication or took only acute medication had an average of 16-17 sick leave days a year, those on preventive migraine treatment (suggestive of chronic and more severe migraine) were on sick leave more than 22 days a year on average. Of note, preventive medications prescribed were not developed for migraine and included beta-blockers, antidepressants, and others4.
The recurrent sick days and health care visits mirror a high unmet need among people with migraine. Especially those suffering from unresponsive treatment or refractory migraine have a need for improved migraine management.
Clinical trials have shown that erenumab, a monoclonal antibody targeting the calcitonin gene-related peptide (CGRP) pathway, reduces the number of monthly migraine days in patients with episodic or chronic migraine1,4.
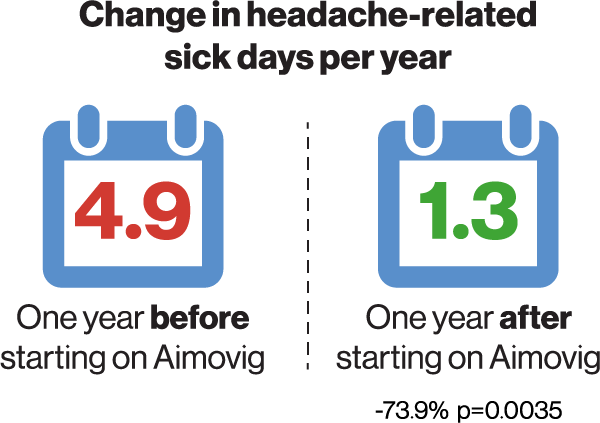
To elucidate whether the clinical trial results could translate into fewer sick leave days and health care visits in a real-world setting, data from 82 patients with migraine who were treated with erenumab were extracted from the Finnish registry of occupational health care users*4. Included patients had at least eight migraine days a month and had failed on two or more preventive treatments. Also, because of the Finnish reimbursement criteria, only patients who responded to erenumab with a ≥50% reduction in monthly migraine days were studied. As compared to one year before initiation of erenumab, the number of migraine-related sick leave days were lowered by 74% one year after erenumab onset4. In exact numbers, the patients went from an average of 4.9 sick leave days per year before erenumab to 1.3 sick leave days annually one year after erenumab4. See Figure. Where about one in three had at least one sick leave day a year due to headache, this proportion decreased to about one in six following the initiation of erenumab4.
The number of health care visits related to headache diminished from 4.9 visits before to 2.7 visits after (p<0.001), corresponding to a reduction of 45%4. This was also reflected in the fraction of patients with five or more annual headache-related visits which decreased from about 40% to about 16% (p<0.001) after initiation of erenumab treatment4.
Change in headache-related sick leave days in patients on erenumab treatment and in age- and gender-matched controls. P value compared to reference (12 months before initiation of treatment)4
Erenumab treatment also led to a diminished need for acute migraine medication. The use of triptans was reduced in 68% of patients before treatment to 48% after the initiation of erenumab (30%, p=0.012) 4, and a similar reduction was seen for drugs commonly used for migraine prophylasxis other than erenumab, from 66% pre-index to 45% post-index (-31%, P=0.0004)4.
In contrast, no changes were observed for other pain medications. In conclusion, the study implies that erenumab may aid in reducing sick days and the need for acute treatment.
Read the full-length articles here:
- doi.org/10.1186/s10194-019-0964-5
- doi.org/10.1007/s40120-021-00303-x
Erenumab Safety profile: Common side effects: hypersensitivity reactions, injection site reactions, constipation, muscle spasms, and pruritus1.
Boka möte |
 |
Håll dig uppdaterad |
Referenser
- Giamberardino MA, et al. Anti-CGRP monoclonal antibodies in migraine: current perspectives. Intern Emerg Med. 2016;11(8):1045-1057.
- De Matteis E, et al. Migraine Prevention with Erenumab: Focus on Patient Selection, Perspectives and Outcomes. Ther Clin Risk Manag. 2022;18:359-378.
- Albanese M & Mercuri NB. Could the New Anti-CGRP Monoclonal Antibodies Be Effective in Migraine Aura? Case Reports and Literature Review. J Clin Med. 2022;11(5):1228.
- Lipton RB, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-9.
- Borsook D, et al. Sex and the migraine brain. Neurobiol Dis. 2014;68:200-14.
- Warfvinge K, et al. Estrogen receptors α, β and GPER in the CNS and trigeminal system - molecular and functional aspects. J Headache Pain. 2020;21(1):131.
- Krause DN, et al. Hormonal influences in migraine - interactions of oestrogen, oxytocin and CGRP. Nat Rev Neurol. 2021;17(10):621-633.
Aimovig (erenumab) (F), Rx, ATC-kod N02CD01, lösning i förfylld injektionspenna för subkutant bruk. Indikation: Migränprofylax hos vuxna som har minst 4 migrändagar per månad när behandling med Aimovig sätts in. Dosering: 70 eller 140 mg var 4:e vecka. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne som anges i Innehåll. Varning och försiktighet: För information se www.fass.se. För ytterligare information om pris och förpackning se www.fass.se. Kontaktuppgifter: Aimovig tillhandahålls av Novartis Sverige AB, www.novartis.se. Vid frågor om våra läkemedel kontakta [email protected]. Datum för översynen av produktresumén: 2024-12-19.
Aimovig subventioneras endast för patienter med kronisk migrän som efter optimerad behandling inte haft effekt av eller inte tolererat minst två profylaktiska läkemedelsbehandlingar. Kronisk migrän definieras som minst 15 huvudvärksdagar per månad i mer än 3 månader varav minst 8 dagar per månad ska ha varit med migränhuvudvärk (enligt ICHD-3). Subventioneras endast vid förskrivning av neurolog eller läkare verksam vid neurologklinik eller klinik/enhet specialiserad på behandling av patienter med kronisk migrän.

