Learnings from 3+ years with CGRP mAbs for migraine prevention
Headache experts from across the Nordics met physically and online on the last day of spring to take stock of more than 3 years of clinical experience with CGRP monoclonal antibodies for migraine prevention. Topics included difficult-to-treat patients and hormonal influences in migraine.
With themes like: long-term efficacy and safety of CGRP mAbs, migraine’s impact on work life, hormonal influences in migraine, and CGRPs in patients with difficult-to-treat migraine, the recent Nordic Migraine Meeting on May 31st, covered a wide field. Yet, focus was on the Nordic clinical experience with CGRP mAbs since launch.
Karl Bjørnar Alstadhaug, a consulting neurologist at Nordland Hospital and professor at the Clinical Institute of Medicine at the Arctic University of Norway, shared his clinical experience on the use of CGRP mAbs in patients with difficult-to-treat (DTT) migraine.
Often, neurology clinics face patients that are far more difficult to treat than patients included in clinical trials, such as patients with medication overuse headache.1 For this reason, clinical trial patients may not always translate to the real-world patients. Nonetheless, for CGRP mAbs, real world data are found to be consistent with clinical trial data.2 This is in line with Karl Bjørnar Alstadhaug’s clinical experience, as he has seen patients with years of DTT migraine who previously have tried multiple preventive treatments without success. Introduction of treatment with CGRP mAbs can have substantial positive impact in these patients’ lives by reducing their nearly daily migraine attacks to around 4-6 migraine days per month.3
Thus, real-world evidence combined with his own clinical experience lead Karl Bjørnar Alstadhaug to categorize CGRP mAbs as 'game changers' in the treatment of migraine, which in his opinion can be used successfully also in difficult to treat refractory patients.
Migraine is 3x more common in women than in men.4 In women, migraine frequency and severity vary during reproductive life and menstrual cycle, suggesting that fluctuations in reproductive hormones influence migraine’s pathophysiology.5 Such hormonal influence in migraine was the topic of Lars Edvinsson’s talk at the Nordic Migraine Meeting. Lars Edvinsson, who is professor of Internal Medicine at Lund University and of Clinical Pharmacology at Copenhagen University, has, together with his colleagues, recently published a hypothesis of hormone withdrawal as a trigger in menstrual migraine.
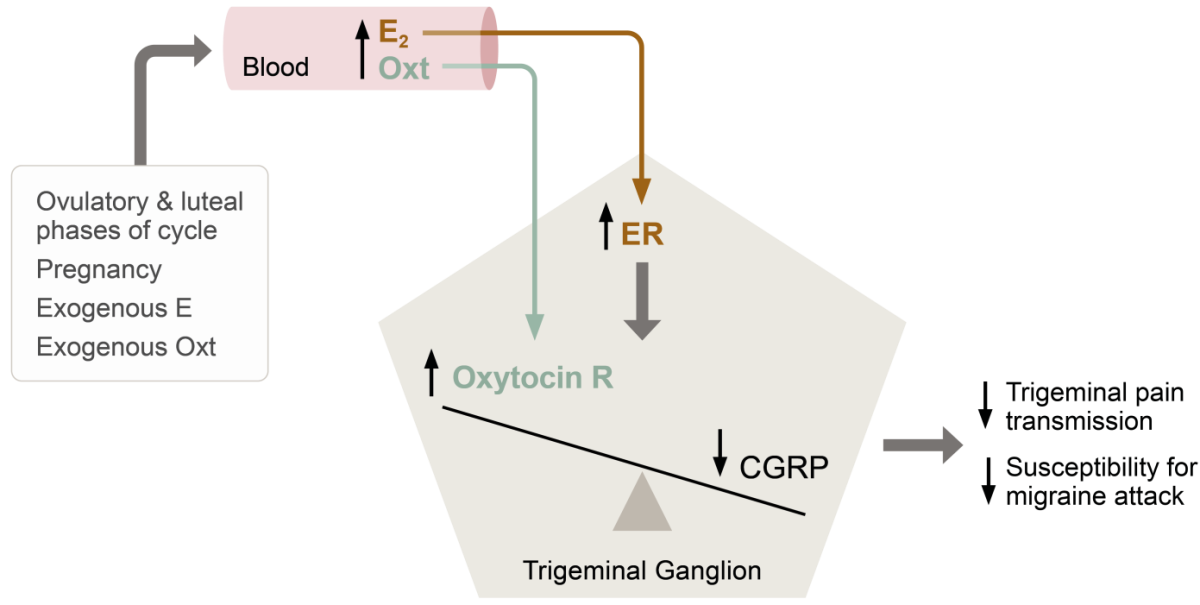
The incidence of migraine in women rises with puberty, peaks during reproductive years, and subsides with menopause, and attacks of migraine are most likely to occur when oestrogen levels decline rapidly, as seen immediately before menstruation and postpartum.5
According to basic research studies conducted by Lars Edvinsson and colleagues, all oestrogen receptor (ER) subtypes (ERα, ERβ and the G-protein coupled ER, GPER) are expressed in migraine-related pathways in the central nervous system (CNS) and the peripheral trigeminal ganglia.6
“In the CNS and in the trigeminal ganglion, the oestrogen receptors co-localize with CGRP, CGRP receptors, oxytocin and oxytocin receptors,” Lars Edvinsson explained.
Especially oxytocin and its receptor are of interest here. Oxytocin, a neuropeptide hormone with wide effects both within and beyond female reproduction, is regulated by oestrogens and has circulating levels mirroring those of oestrogen.7
“Data suggest that oxytocin can prevent attacks of migraine. We therefore ask the question whether oxytocin might be the link between oestrogen fluctuations and migraine susceptibility,” Lars Edvinsson argued.
Thus, extensive co-localization of relevant hormones, neuropeptides and receptors in areas central to migraine coupled with clear epidemiological and clinical evidence of a hormonal influence in the disease has lead Lars Edvinsson and co-workers to suggest a model for, how withdrawal of oestrogen can trigger migraine (see Figure).7
| High levels of oestrogen and oxytocin | Low levels of oestrogen and oxytocin |
|---|---|
 |
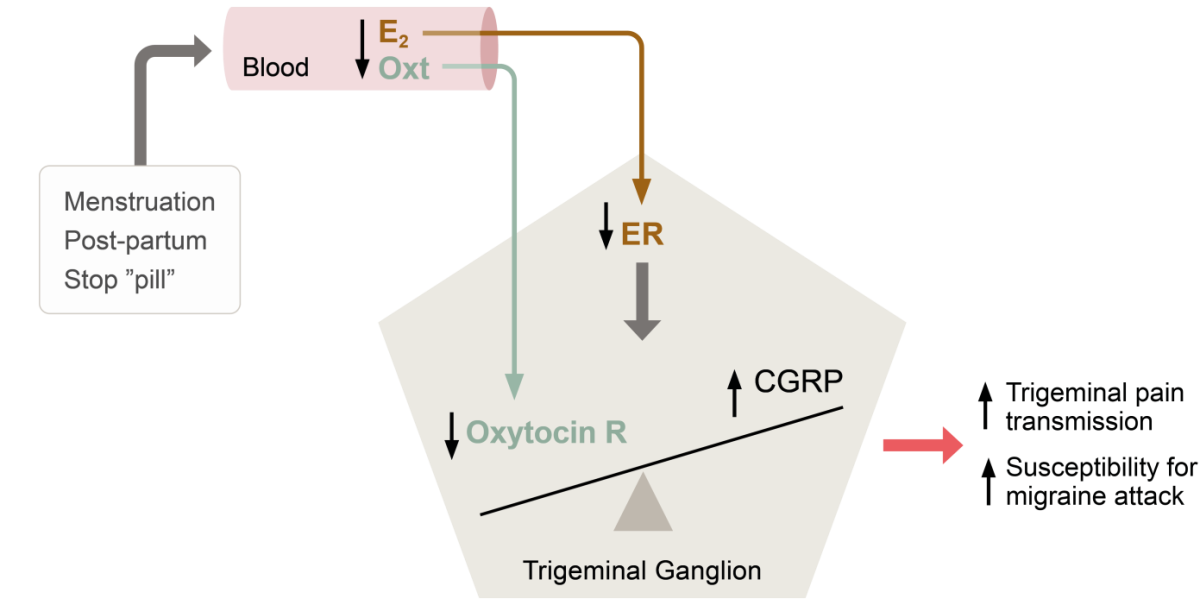 |
Theory of hormonal balance in the trigeminal ganglion and the impact of hormone withdrawal in menstrual migraine. Abbreviations: E2, oestrogen; Oxt, oxytocin; CGRP, calcitonin gene-related peptide; ER, oestrogen receptor; R, receptor. Based on Krause et al, 2021.7
Use of CGRP mAbs in specific subpopulations of menstrual migraine patients has not yet been investigated in clinical trials. According to Lars Edvinsson, further research within this area is warranted including the potential effects of ER agonists and oxytocin.
Boka möte |
 |
Håll dig uppdaterad |
Referenser
- Giamberardino MA, et al. Anti-CGRP monoclonal antibodies in migraine: current perspectives. Intern Emerg Med. 2016;11(8):1045-1057.
- De Matteis E, et al. Migraine Prevention with Erenumab: Focus on Patient Selection, Perspectives and Outcomes. Ther Clin Risk Manag. 2022;18:359-378.
- Albanese M & Mercuri NB. Could the New Anti-CGRP Monoclonal Antibodies Be Effective in Migraine Aura? Case Reports and Literature Review. J Clin Med. 2022;11(5):1228.
- Lipton RB, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-9.
- Borsook D, et al. Sex and the migraine brain. Neurobiol Dis. 2014;68:200-14.
- Warfvinge K, et al. Estrogen receptors α, β and GPER in the CNS and trigeminal system - molecular and functional aspects. J Headache Pain. 2020;21(1):131.
- Krause DN, et al. Hormonal influences in migraine - interactions of oestrogen, oxytocin and CGRP. Nat Rev Neurol. 2021;17(10):621-633.
Aimovig (erenumab) (F), Rx, ATC-kod N02CD01, lösning i förfylld injektionspenna för subkutant bruk. Indikation: Migränprofylax hos vuxna som har minst 4 migrändagar per månad när behandling med Aimovig sätts in. Dosering: 70 eller 140 mg var 4:e vecka. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne som anges i Innehåll. Varning och försiktighet: För information se www.fass.se. För ytterligare information om pris och förpackning se www.fass.se. Kontaktuppgifter: Aimovig tillhandahålls av Novartis Sverige AB, www.novartis.se. Vid frågor om våra läkemedel kontakta [email protected]. Datum för översynen av produktresumén: 2024-12-19.
Aimovig subventioneras endast för patienter med kronisk migrän som efter optimerad behandling inte haft effekt av eller inte tolererat minst två profylaktiska läkemedelsbehandlingar. Kronisk migrän definieras som minst 15 huvudvärksdagar per månad i mer än 3 månader varav minst 8 dagar per månad ska ha varit med migränhuvudvärk (enligt ICHD-3). Subventioneras endast vid förskrivning av neurolog eller läkare verksam vid neurologklinik eller klinik/enhet specialiserad på behandling av patienter med kronisk migrän.

